[ad_1]
Abstract
- Firm Announcement Date:
- November 12, 2018
- FDA Publish Date:
- June 06, 2019
- Product Kind:
- Medicine
- Purpose for Announcement:
- Firm Title:
- Oscor Inc.
- Model Title:
- Product Description:
-
Product Description
Short-term Bipolar Pacing Lead, Mannequin TB
Firm Announcement
On September 26, 2018 Oscor notified prospects of a recall for sure tons (Recall No. 1035166- 09/07/2018-01-R) of TB Unshrouded Bipolar Pacing Leads. As a part of the recall correction actions, Oscor is retrieving any remaining stock out within the subject. The recall scope is being expanded to incorporate expired stock for gadgets distributed between December 21, 2011 to Might 17, 2018. The recall growth is to make sure correct disposition of expired models. The FDA has been notified and is conscious Oscor Inc. is voluntarily taking this motion.
INTENDED USE:
The Short-term Bipolar Pacing Lead, Mannequin TB is used transvenously for momentary pacing and sensing of the guts together with a appropriate exterior pulse generator.
DEVICE DESCRIPTION:
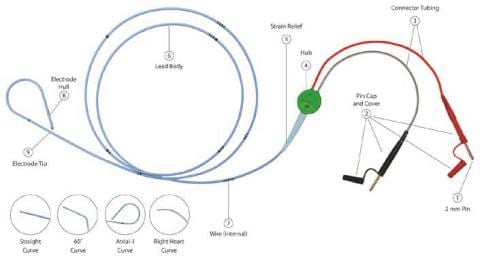
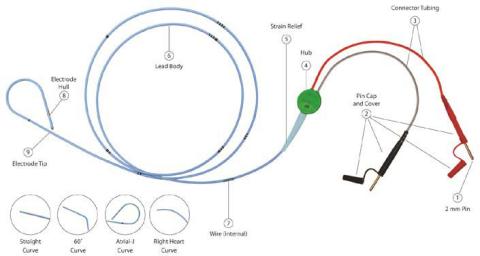
The momentary pacing lead is a bipolar lead with depth markings. The proximal finish of the lead contains two 2mm (unshrouded) connectors which match on to most pulse mills or extension cable. The French dimension is printed on the lead hub.
REASON FOR THE VOLUNTARY PRODUCT REMOVAL:
Throughout the usage of some TB – Short-term Bipolar Pacing Leads, that includes the 2mm unshrouded connectors, the connector cap housing (see Image 1, No. 2 Pin Cap and Cowl) might slide and probably expose the connection wire. In some cases, this will trigger the wire to be extra vulnerable to lack of connectivity or breakage throughout motion of the cables inflicting interruption of the pacing system. The evaluation of the returned gadgets attributed the failure to a design change of the cap housing of the pins. Within the final six years, a complete of 4 critical accidents had been reported to Oscor which had been attributed to a connector cap malfunction inflicting the lead connector to separate throughout use probably resulting in an interruption of the pacing system. No deaths had been reported; nonetheless the danger for critical harm and/or dying is a priority if the connectors separates throughout use.
EVENT DESCRIPTION: Throughout the usage of some TB – Short-term Bipolar Pacing Leads, that includes the 2mm unshrouded connectors, the connector cap housing (see Image 1, No. 2 Pin Cap and Cowl) might slide and probably expose the connection wire. In some cases, this will trigger the wire to be extra vulnerable to lack of connectivity or breakage throughout motion of the cables inflicting interruption of the pacing system.
REASON FOR RECALL: Within the final six years, a complete of 4 critical accidents had been reported to Oscor which had been attributed to the above connector cap malfunction. No deaths had been reported; nonetheless the danger for critical harm and/or dying is a priority if the connectors separates throughout use.
WARNING:
For pacing dependent sufferers, an interruption of pacing system may end in critical harm or dying if not detected. Steady monitoring is required.
MODEL NUMBERS:
| GTIN | Mannequin Quantity | Description | Specs | |
|---|---|---|---|---|
| French dimension | Curve Kind | |||
| 00836559009726 | 020004 | TB LEAD 4F UN-SHROUDED | 4F | Straight |
| 00836559009733 | 020005 | TB LEAD 5F UN-SHROUDED | 5F | Straight |
| 00836559009740 | 020006 | TB LEAD 6F UN-SHROUDED | 6F | Straight |
| 00836559009788 | 020010 | TB LEAD 4F UN-SHROUDED | 4F | Atrial J |
| 00836559009795 | 020011 | TB LEAD 5F UN-SHROUDED | 5F | Atrial J |
| 00836559009801 | 020012 | TB LEAD 6F UN-SHROUDED | 6F | Atrial J |
| 00836559009856 | 020017 | TB LEAD 5F UN-SHROUDED | 5F | 60° Curve |
| 00836559009863 | 020018 | TB LEAD 6F UN-SHROUDED | 6F | 60° Curve |
| 00836559009900 | 020022 | TB LEAD 4F UN-SHROUDED | 4F | Proper Coronary heart |
| 00836559009917 | 020023 | TB LEAD 5F UN-SHROUDED | 5F | Proper Coronary heart |
| 00836559009924 | 020024 | TB LEAD 6F UN-SHROUDED | 6F | Proper Coronary heart |
| 00836559009030 | TBK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Straight |
| 00836559009054 | TBK05110USG | TB LEAD 5F UN-SHROUDED CONVENIENCE KIT | 5F | Straight |
| 00836559009078 | TBK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | Straight |
| 00885672007027 | TBVK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | 60° Curve |
| 00885672007034 | TBJK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Atrial J |
| 00885672004378 | TBRHK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Proper Coronary heart |
| 00885672103682 | TBRHK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | Proper Coronary heart |
| The Fashions listed under weren’t a part of the unique press launch issued by Oscor Inc. on September 24, 2018. | ||||
| 00836559009849 | 020016 | TB LEAD 4F, UNSHOURDED | 4F | 60° Curve |
| 00885672004354 | TBVK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | 60° Curve |
| 00885672004392 | TBRHK05110USG | TB LEAD 5F UN-SHROUDED CONVENIENCE KIT | 5F | Proper Coronary heart |
Buyer might contact Oscor’s Buyer Relations Group, Monday to Friday from 8:30AM to five:30PM Japanese Time at 727-937-2511 or through electronic mail at [email protected].
Healthcare professionals are inspired to report any malfunction and/or antagonistic occasions associated to the usage of these merchandise to the FDA’s MedWatch Security Info and Antagonistic Occasion Reporting Program:
Full and submit the report On-line: www.fda.gov/MedWatch/report.htm
Download form or name 1-800-332-1088 to request a reporting kind, then full and return to the deal with on the pre-addressed kind, or submit by fax to 1-800-FDA-0178.
Product Photographs
[ad_2]
Source link