The CDC confirmed on February 13, 2026 that Rosabella-brand moringa capsules are linked to the first outbreak of Salmonella carrying the NDM-1 gene ever documented in the United States. Seven people across seven states got sick. Three were hospitalized. Nobody has died, but the drug resistance profile of this strain has public health officials raising alarms well beyond a typical supplement recall.
The Rosabella moringa recall involves a Salmonella Newport strain resistant to every first-line antibiotic used to treat Salmonella infections, and most of the alternatives. The FDA classified it as “extensively drug-resistant” (XDR). The pathogen carries NDM-1. This gene destroys carbapenems, the antibiotics doctors turn to when everything else fails, and the WHO classifies it as a “critical priority” threat.
Moringa oleifera is a tropical plant sold as a “superfood” supplement in capsule and powder form. Marketed for energy, immune support, and gut health, moringa has gained massive traction on TikTok and other social platforms. In this case, consumers paying for a wellness product received a pathogen that defeats nearly every antibiotic available.
What Is the Rosabella Moringa Recall?
On February 13, 2026, the FDA announced an investigation into Rosabella-brand moringa powder capsules distributed by Ambrosia Brands LLC. The agency recommended the company recall 52 lot codes after lab testing confirmed extensively drug-resistant Salmonella Newport carrying the NDM-1 carbapenemase gene. Ambrosia Brands agreed to a voluntary recall.
Seven confirmed illnesses have been reported across Arizona, Florida, Iowa, Illinois, Indiana, Tennessee, and Washington. Illness onset dates range from November 7, 2025 to January 8, 2026. Three patients required hospitalization. No deaths have been reported.
The FDA “recommended” the recall. The company “agreed.” The FDA gained mandatory recall authority under the 2011 Food Safety Modernization Act, but has used it only once for any food product, and never for a traditional dietary supplement. In practice, the system still runs on voluntary cooperation.
Recalled Lot Codes and Products
The recall covers Rosabella moringa powder capsules packaged in white plastic bottles with green labels. Fifty-two lot codes are affected, with expiration dates spanning March 2027 through November 2027.
How to find your lot code: check the bottom of the bottle. The lot code is the middle seven digits of the code printed above the expiration date. If your lot code falls between 5020591 (expiration March 2027) and 5100048 (expiration November 2027), your product is included in the recall.
The full list of recalled lot codes is on the FDA investigation page linked above.
Where Were Rosabella Moringa Capsules Sold?
Rosabella capsules were sold through five channels:
- Amazon
- eBay
- Shein
- TikTok Shop
- Tryrosabella.com (the company’s own website)
That distribution mix is telling. None of these platforms perform pre-sale ingredient testing or safety verification for supplements. A product can go from production to sale in days, faster than any regulatory review could catch up.
Why Is This Salmonella Outbreak Different?
About 1.35 million Americans contract Salmonella every year, according to the CDC. Most recover on their own within four to seven days without antibiotics. When treatment is needed (typically for severe diarrhea, bloodstream infections, or high-risk patients), doctors reach for ciprofloxacin, ceftriaxone, or azithromycin. These drugs work fast and they’re widely available.
The Rosabella moringa strain resists all of them.
What Is XDR Salmonella?
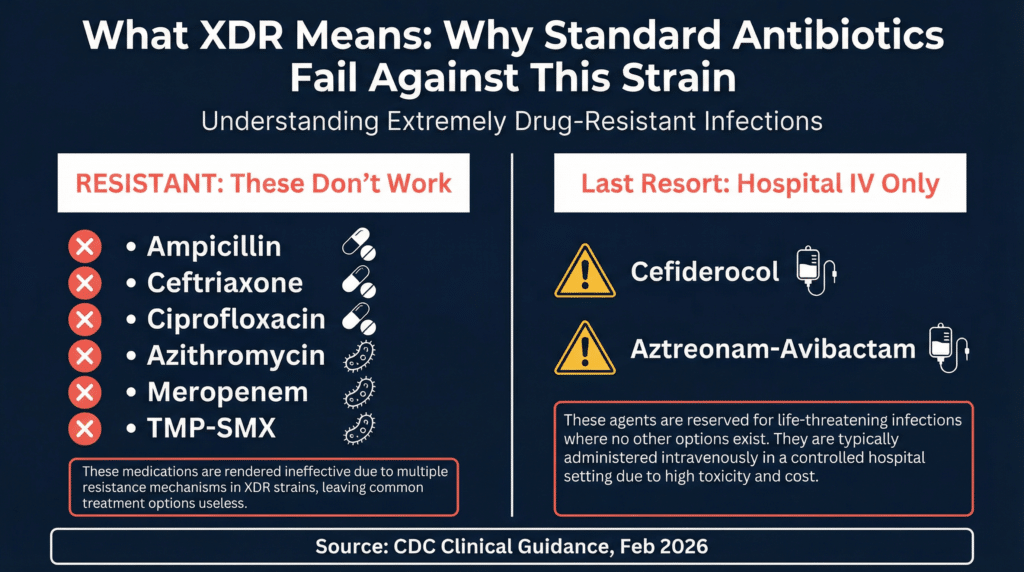
XDR stands for “extensively drug-resistant.” In Salmonella, that means the bacteria resist all first-line antibiotics (ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, fluoroquinolones, and third-generation cephalosporins) and the most commonly recommended alternatives.
The CDC’s assessment is blunt: this strain is “resistant to all first-line and alternative antibiotics commonly recommended for the treatment of Salmonella infections.”
What remains? A handful of last-resort drugs that most hospitals don’t stock, that require IV infusion, and that cost thousands of dollars per treatment course.
“Resistant to all first-line and alternative antibiotics commonly recommended for the treatment of Salmonella infections.”
CDC, February 2026
What Is NDM-1 and Why Do Health Officials Call It a Nightmare?
NDM-1 (New Delhi metallo-beta-lactamase 1) is a gene that produces an enzyme capable of destroying carbapenem antibiotics, the drugs doctors treat as the final line of defense against resistant infections. When a bacterium carries NDM-1, it can survive exposure to nearly every antibiotic in the standard medical toolkit.
What makes NDM-1 especially dangerous is how it spreads. The gene sits on a plasmid — a small, mobile piece of DNA that bacteria swap between each other, even across different species. An NDM-1-carrying Salmonella in a person’s gut could transfer that resistance gene to E. coli, Klebsiella, or other bacteria already living there. This transfer can happen within hours.
First identified in 2008 in a patient hospitalized in New Delhi, NDM-1 has since been detected in more than 100 countries and at least 84 genetic variants. The CDC reported in September 2025 that NDM-CRE infections in the U.S. surged 460% between 2019 and 2023. But until now, NDM-1 had remained largely a hospital problem, turning up in patients who’d received medical care abroad or in healthcare settings.
The Rosabella outbreak changed that. This is the first time NDM-1 has appeared in a pathogen linked to a consumer product sold to the general public.
What Happens If You Took Recalled Rosabella Moringa?
Not everyone who ingested contaminated capsules will develop symptoms. Salmonella affects people differently depending on the dose consumed, immune status, age, and individual biology. The CDC estimates that for every confirmed Salmonella case, roughly 29 more go undiagnosed: people who get sick, recover at home, and never see a doctor.
That said, this is not a strain to wait out.
Symptoms of XDR Salmonella Infection
Symptoms typically begin 6 hours to 6 days after exposure and include:
- Diarrhea (sometimes bloody)
- Fever
- Stomach cramps
- Nausea and vomiting
- Headache
Standard Salmonella infections usually resolve within 4–7 days. XDR infections can last longer because the body is fighting without pharmaceutical backup. Data from a large XDR Salmonella outbreak in Pakistan found that XDR patients had a 38% complication rate compared to 18% for non-resistant strains, and took a median of 20 days before hospitalization versus 13 days for non-XDR cases. Mortality trended higher in the XDR group (1.8% vs. 0.6%), though the difference did not reach statistical significance in that study.
When to See a Doctor
Contact a healthcare provider if you:
- Took Rosabella moringa capsules and develop any symptoms listed above
- Have diarrhea lasting more than 3 days
- Run a fever above 102°F (39°C)
- Show signs of dehydration (dark urine, dizziness, dry mouth)
- Are immunocompromised, pregnant, over 65, or under 5
Tell your doctor you consumed a product linked to drug-resistant Salmonella. It will change which antibiotics they prescribe and how quickly they order susceptibility testing. Without that context, a clinician will likely start with ceftriaxone (standard of care), which won’t work against this strain.
How Doctors Treat XDR Salmonella — And Why It’s Harder Than You Think
When standard antibiotics fail, doctors are left with a short and expensive list. The CDC’s clinical guidance for this specific strain identifies three potential treatment options, while noting that evidence-based recommendations do not yet exist:
Cefiderocol (Fetroja): A specialized antibiotic that bypasses NDM-1 resistance by hijacking the bacteria’s iron transport system. It requires IV infusion every 8 hours, is primarily available at major medical centers, and costs $3,000 to $16,000 per treatment course.
Aztreonam-avibactam: A combination of two separately marketed drugs used off-label together. Generally available only at tertiary care centers with infectious disease specialists on staff. Treatment courses can exceed $5,000 to $10,000+.
Fosfomycin: An option for uncomplicated infections, but the oral form has poor absorption. The IV form, which works better for serious cases, is not FDA-approved in the United States.
The practical problem is access. Community hospitals, where most people first seek care, often lack the specialized testing needed to rapidly identify NDM-producing bacteria, and may not stock these last-resort drugs. Patients may spend 3–5 days on ineffective antibiotics while waiting for culture results. Some may need transfer to a larger medical center. Every hour of delay in appropriate treatment increases risk.
Is Rosabella Moringa Made in the USA?
Rosabella moringa capsules are distributed by Ambrosia Brands LLC, a company registered in Wyoming. But “registered in Wyoming” and “manufactured in the USA” are very different things.
Ambrosia Brands lists two addresses, one in Cheyenne and one in Sheridan. Both are commercial registered agent locations, not manufacturing facilities or offices. Wyoming requires minimal disclosure and charges about $104 to form an LLC. The state doesn’t require owners to publicly identify themselves. It’s one of the easiest states in the country to set up an anonymous shell company.
The company is roughly two years old (incorporated February 2024), carries a C rating from the Better Business Bureau, and has accumulated 245 BBB complaints, a high number for a company that young. Where the moringa itself comes from remains under investigation. The FDA’s traceback has not yet identified the contamination source. Alibaba wholesale listings reference “Rosabella Moringa” capsules in OEM manufacturing contexts, pointing toward overseas white-label production.
How Social Commerce Sells Unvetted Supplements
Rosabella moringa was sold on TikTok Shop, Shein, Amazon, and eBay. Sellers on these platforms self-certify compliance and no pre-sale safety testing exists. A supplement company can go from concept to nationwide distribution in days, outrunning any regulatory process.
The moringa hashtag has accumulated billions of views on TikTok. Rosabella ran “Moringa Cleanse” campaigns encouraging multi-day cleanses. Content on Lemon8 recommended adding Rosabella moringa to drinks, including posts directed at pregnant women. The algorithm amplifies what’s trending, and wellness supplements trend constantly, creating a pipeline where tens of thousands of units ship before regulators know the product exists.
The Two Moringa Outbreaks, Explained
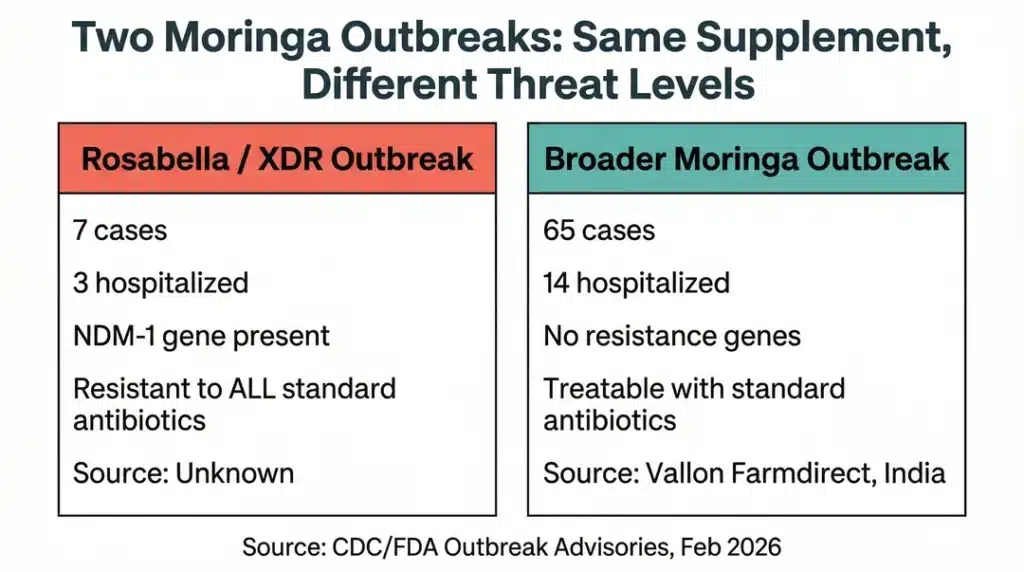
The Rosabella recall is separate from a larger moringa-linked Salmonella outbreak that has sickened at least 65 people across 28 states since mid-2025. The two outbreaks involve different strains with completely different threat levels.
| Rosabella / XDR Outbreak | Broader Moringa Outbreak | |
|---|---|---|
| Strain | Salmonella Newport with NDM-1 gene (XDR) | Salmonella Typhimurium & Newport (not drug-resistant) |
| Confirmed cases | 7 across 7 states | 65 across 28 states |
| Hospitalizations | 3 | 14 |
| Drug resistance | Resistant to all standard antibiotics | No predicted resistance |
| Products | Rosabella moringa capsules | Live it Up Super Greens, Why Not Natural, Member’s Mark, others |
| Supplier | Ambrosia Brands LLC (source unknown) | Traced to Vallon Farmdirect PVT LTD, Jodhpur, India |
The broader outbreak was traced to a single lot from an Indian supplier. The Rosabella contamination source has not been identified. Traceback is ongoing. The broader outbreak was traced to a single lot from an Indian supplier. The Rosabella contamination source has not been identified. Traceback is ongoing.
Two different strains. Two different threat levels. One shared lesson: the moringa supplement supply chain has systemic contamination problems that a single recall won’t fix.
Why Supplement Recalls Still Depend on Voluntary Cooperation
The Dietary Supplement Health and Education Act of 1994 (DSHEA) created the regulatory framework that made this outbreak possible. Under DSHEA:
- Supplements do not require FDA pre-market approval for safety or efficacy
- The manufacturer, not the FDA, is responsible for verifying product safety
- The FDA can only act after people get sick
- Recalls remain functionally voluntary; the FDA has mandatory recall authority under FSMA but has exercised it only once for any food product, and never for a traditional supplement
The FDA itself has acknowledged the gap, noting that “companies can often introduce a dietary supplement to the market without notifying the FDA.”
Since DSHEA passed, the number of supplement products on the U.S. market has grown from roughly 4,000 to more than 80,000. Americans spend an estimated $60 billion or more per year on supplements. The FDA inspects a fraction of what’s sold. Multiple reform efforts, including the Dietary Supplement Listing Act (which would require pre-market registration), have never reached a floor vote.
In the broader moringa outbreak, five months elapsed between the first reported illness and the first recall. That timeline isn’t unusual under the current system. It’s the system working exactly as designed.
What to Do If You Have Rosabella Moringa Capsules
- Check the lot code on the bottom of the bottle — it’s the middle seven digits printed above the expiration date
- If your lot code falls between 5020591 and 5100048, stop taking the product immediately
- Keep the bottle for reference in case you develop symptoms later
- If you’ve taken the capsules and feel fine, monitor yourself for up to 6 days after your last dose
- If you develop diarrhea, fever, or stomach cramps, tell your doctor you consumed a product linked to XDR Salmonella
- Report adverse reactions to the FDA’s MedWatch program or call 1-800-FDA-1088
- Contact Ambrosia Brands at (646) 854-8686 or [email protected] for refund information
For the latest case counts and investigation updates, check the CDC’s outbreak advisory or the FDA’s investigation page.
What This Outbreak Signals for Antibiotic Resistance
Seven confirmed cases may sound small. But the mechanism of resistance matters more than the case count here.
NDM-1 sits on a plasmid, a piece of DNA built for sharing. When a person ingests NDM-1-carrying Salmonella, those bacteria enter the gut, where trillions of other bacteria live. The resistance gene can jump to resident gut flora, potentially creating a long-term reservoir of carbapenem-resistant bacteria in an otherwise healthy person. That reservoir doesn’t cause illness on its own, but it means the next infection that person gets may be harder to treat.
Now consider the scale. A contaminated supplement shipped to five retail platforms nationwide. The CDC’s own 29:1 undercount ratio suggests the true number of exposures may be far higher than seven confirmed cases.
The WHO estimated that antimicrobial resistance directly caused 1.27 million deaths globally in 2019 and and was associated with a total of 4.95 million deaths when including cases where resistance was a contributing factor. The O’Neill Report projects 10 million annual deaths by 2050 if resistance trends continue — a number that would exceed cancer. Those projections have always felt abstract.
A moringa capsule purchased on TikTok Shop carrying the NDM-1 gene makes them less so.
Before this outbreak, NDM-1 was primarily a hospital problem in the U.S., arriving through medical tourism and healthcare transmission. Finding it in a consumer supplement sold on Shein and Amazon represents something new: antibiotic resistance entering the consumer food supply through an unregulated product.
CDC epidemiologist Danielle Rankin warned in September 2025: “This sharp rise in NDM-CRE means we face a growing threat that limits our ability to treat some of the most serious bacterial infections.”
The Rosabella moringa outbreak is that threat arriving exactly where public health officials feared — outside the hospital, inside a product marketed for wellness.
Frequently Asked Questions
Is Rosabella moringa safe to take right now?
No. All Rosabella moringa capsules with lot codes between 5020591 and 5100048 are part of an active recall linked to extensively drug-resistant Salmonella. Stop taking the product, keep the bottle, and monitor for symptoms. Contact Ambrosia Brands at (646) 854-8686 for refund information.
What does “extensively drug-resistant” (XDR) mean?
XDR means the Salmonella strain resists all first-line antibiotics and most alternatives used to treat the infection. In this case, the strain also carries the NDM-1 gene, which destroys carbapenems, the antibiotics typically reserved as the last line of defense. Treatment requires specialized IV drugs available mainly at major medical centers.
Is the Rosabella recall the same as the broader moringa recall?
No. The Rosabella outbreak (7 cases, XDR Salmonella with NDM-1) is separate from the larger moringa-linked outbreak (65+ cases, non-resistant Salmonella). The broader outbreak was traced to brands like Live it Up Super Greens and Why Not Natural, sourced from a supplier in Jodhpur, India. The Rosabella contamination source is still under investigation.
Can drug-resistant Salmonella kill you?
Most healthy adults recover from Salmonella without antibiotics. But XDR strains raise the risk. Data from a large XDR Salmonella outbreak in Pakistan showed a mortality rate of 1.8% for XDR cases versus 0.6% for non-resistant cases, though that difference was not statistically significant. Immunocompromised individuals, young children, and elderly adults face higher risk. If you develop symptoms after taking recalled moringa, seek medical care promptly.