Summary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason Description
Presence of Particulate Matter
- Company Name:
- AuroMedics Pharma LLC
- Brand Name:
- Product Description:
-
Product Description
Polymyxin B for Injection USP, 500,000 Units/Vial
Company Announcement
FOR IMMEDIATE RELEASE – January 26, 2022 – East Windsor, New Jersey, AuroMedics Pharma LLC has initiated a voluntary recall of lot number CPB200013 of Polymyxin B for Injection USP, 500,000 Units/Vial, to the consumer level from the USA market due to a product complaint for the presence of particulate matter, identified as hair being discovered in a vial within this lot.
Risk Statement: The administration of an intravenous product containing hair, even with the use of a filter, could cause a patient to experience serious hypersensitivity reactions that may be life- threatening. To date, AuroMedics Pharma LLC has not received reports of any adverse events or identifiable safety concerns attributed to the product consumed from this lot.
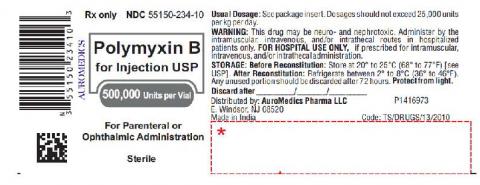
Polymyxin B for Injection USP is a sterile, white lyophilized cake or powder, suitable for preparation of sterile solutions for intramuscular, intravenous, intrathecal, or ophthalmic use indicated in the treatment of infections or the urinary tract, meninges (membranes that protect the brain and spinal cord), and bloodstream caused by susceptible strains of bacteria. It is packaged in a carton containing vials for Parenteral or Ophthalmic Administration, NDC 55150-234-10. The affected Polymyxin B for Injection lot being recalled is CPB200013 with an expiration date of 09/2022. AuroMedics shipped the entire lot to wholesalers nationwide from March 19, 2021, through June 14, 2021.
The product label is as shown below:
AuroMedics Pharma LLC is notifying its distributors by recall letters and is arranging for return/replacement of all recalled product. Consumers/distributors/retailers that have the product lot which is being recalled should immediately stop using and return to place of purchase/contact their doctor as appropriate.
Consumers with medical questions regarding this recall or to report an adverse event can contact AuroMedics Pharma LLC, from 8:00 am to 5:00 pm M-F EST at:
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
If you have any general questions regarding the return of this product, please contact Qualanex at 1-888-280-2046 or email [email protected] (live calls received 7:00 am to 4:00 pm M-F CST).
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.